Neurofeedback training for Autism - Research
- Nov 19, 2022
- 8 min read
Updated: Jan 4
By Anna Jusek
Following general screening of Neurofeedback training for ASD, here you will find further scientific screening addressing the brain patterns that have been shown by research to improve symptoms in ASD following Neurofeedback training.
Due to the complexity of ASD, e.g., the vast array of brain regions and mechanisms associated with the pathology, several neurofeedback (NFT) protocols are investigated.
Connectivity
The number of global brain abnormalities prompts issues in neural connectivity within the form of both hyper and hypoconnectivty. For example, there is short-range overconnectivity, predominantly within the left insula, frontal and pericentral brain regions [3,11,12], and impaired long-range connectivity between the frontal lobe and other cortical regions [7,3]. I
t is suggested that early developmental neuroinflammatory reactions disrupt frontal microcircuitry, provoking brain overgrowth which hinders connectivity between various information processing mechanisms, e.g., diminished connectivity between the frontal lobe and other systems. At the same time, this brain expansion that results in neuropil space reduction prompts excessive connectivity within the frontal lobes.
These structural and functional changes induce developmental stunting as they prohibit further growth in cerebral connectivity and specialisation. Problematic, as the frontal regions are vital in linguistic, cognitive, emotional control and social processes [13]. Supporting research has presented that impaired information flow between frontal and posterior regions slows down response time and negatively impacts executive processing in both visual and spatial domains (especially information involving facial perceptions and working memory) [14].
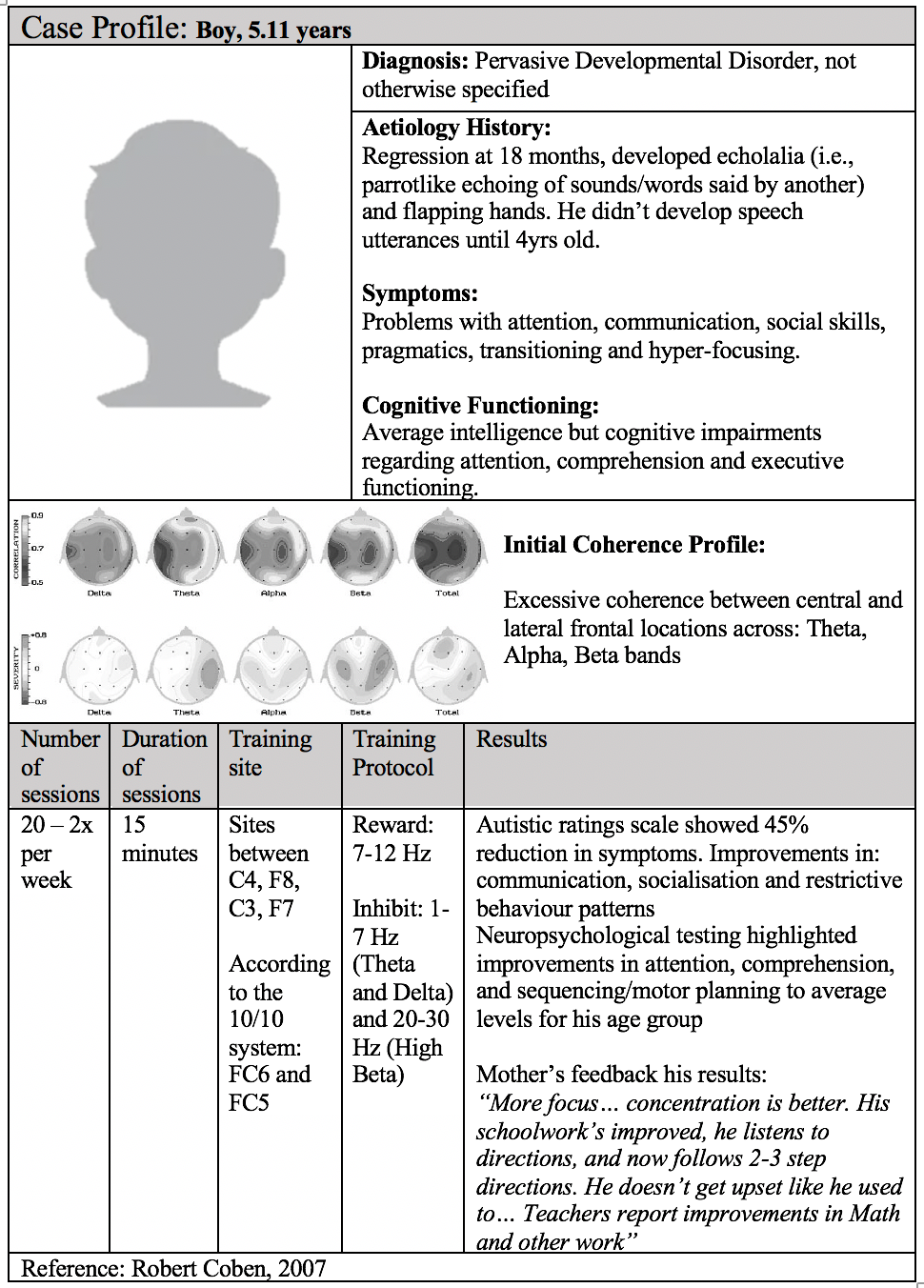
Coben et al. 2007 demonstrated that children with autism displayed greater alpha and beta coherence but less interhemispheric and intra-hemispheric asymmetry [3]. Thus, they theorised that normalising connectivity should improve autistic symptoms.
The results demonstrated that personalised protocols based on connectivity-guided neurofeedback led to improved connectivity and a 45% reduction in autistic symptoms.
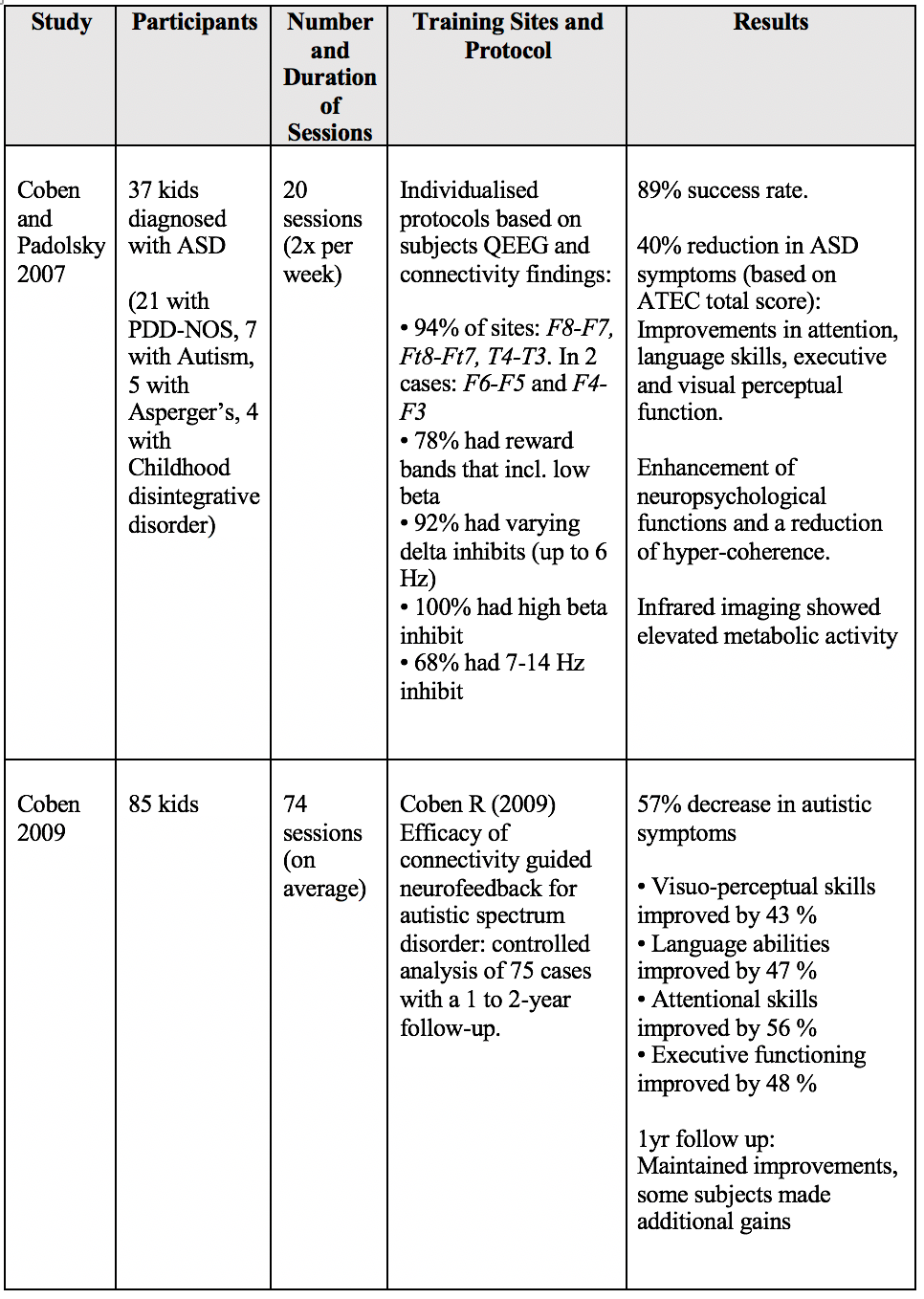
These findings have been replicated. For example, Coby and Padolsky 2007 obtained a 40% reduction in autistic symptoms and hypercoherence, followed by an enhancement in neuropsychological functions after 20 sessions [1].
Neurofeedback appears promising in connectivity, as it uses operant conditioning to exercise and strengthen desired network connections and diminish undesired connections.
Replications:

Low frequency bands: Decrease theta & increase SMR
Low-frequency waves are also targeted in neurofeedback, as attenuated cortical excitability with excessive slow-wave activity (within the frontal regions) and a diminished alpha power band are observed in ASD [15]. Kouijzer et al. carried out several studies in this domain after past research demonstrated that increasing low beta and decreasing theta attenuates ASD symptomology and promotes brain flexibility via; 1. enhancing medial prefrontal brain activation and 2. enhancing the flexibility of the default mode network’s activation, which improves executive functions [16].

The team found that inhibiting theta and increasing beta activation reduced the heightened theta/beta ratio and improved ASD symptomology.
What is significant about the improvements is that they were not observed in the age, sex and IQ-matched control group.
Additionally, the improvements in executive functioning that occurred immediately after the neurofeedback treatment either persisted or further improved 12 months after training [17].
Functions that further improved following NFT treatment entailed motor response inhibition and sustained auditory selective attention. Additionally, improvements in social behaviour (social interaction skills, communicative abilities and typical behaviour) were maintained 12 months onwards. Supporting studies have demonstrated similar promising results [4,18]:

However, Mekkawy found no improvements in five patients (mean age of 10.7yrs) and suggested that earlier intervention may enhance NFT-treatment outcome [18].
Underlying mechanisms that support effective executive functioning and social behaviour include the default mode network, which entails the anterior cingulate cortex (ACC). In addition to generating significant levels of theta activity, the ACC regulates cognitive and emotional processes. During rest, the ACC has a high default metabolism, whereas when other brain regions are activated during cognitive tasks, the ACC deactivates [34].
Kennedy et al. found that ASD subjects did not deactivate their ACC to allow for the activation of other task-related brain regions [35]. Thus, learning to reduce ACC theta activity via NFT allows for increased ACC flexibility, i.e., for the ACC to activate and deactivate appropriately in response to other cognitive and executive demands, which enhances performance.
Mu rhythm training
As previously mentioned, social deficits in ASD are suggested to involve the human mirror neuron system. In normal conditions, mu power around the sensorimotor cortex is suppressed during the observation, imagination and execution of body movements. Mu suppression is accompanied by the activation of regions linked to the hMNS. In ASD however, it has been observed that mu rhythm is absent during the observation of body movements unless the observed or imitated person is familiar to the individual with ASD. This finding was promising as it suggested that mu-rhythm is malleable in ASD [19].
Datko et al. performed 20 hours of mu-rhythm neurofeedback targeting the sensorimotor cortex in children. When assessing the results via fMRI, the ASD group displayed increased activation in brain regions containing the hMNS, e.g., the inferior parietal lobe (IPL). The IPL is involved in sensorimotor integration, perception and performance of goal-directed actions. Thus, increased IPL activation suggests that Neurofeedback treatment enhanced visuomotor integration during imitation. These brain changes were accompanied by behavioural improvements.

Previous studies on mu rhythm training have presented similar positive outcomes [20,21]. The overall results suggest that mu-training increased hMNS activation and thereby induced positive changes in behaviour.

Criticisms of Neurofeedback in Autism
Holtmann et al. 2011, critiqued that research presenting positive ASD-neurofeedback outcomes may not be demonstrating improvements in ASD but in its comorbid disorder, ADHD [22]. Nonetheless, the team stated that due to the high number of individuals with ASD and comorbid ADHD (at the time of the paper, 40-50% of ASD individuals had ADHD), ADHD treatment should be obligatory as it impacts ASD presentation. However, in recent years, meta-analyses have been used to assess the efficacy of neurofeedback on ASD.
Van Hoogdalem et al. 2021 analysed 587 articles (containing 443 participants) and found that 94% of the nonrandomised controlled trials produced positive neurofeedback results on ASD. When only randomised controls were assessed, the efficacy of neurofeedback training increased [23].
Similar to Kouijzer et al. 2009 and Pineda et al. 2014, Datko et al. 2017 found no corresponding changes in their control group, indicating that the benefits of sensorimotor mu-NFT specifically benefit ASD. These findings are significant as they demonstrate that some NFT protocols specifically target maladaptive alterations found only in ASD, disproving Holtmann et al. 2011.
Targeting ASD symptoms
While the therapeutic potential of NFT on autism requires more research, there is a significant amount of research on NFT’s therapeutic effects on the symptoms associated with autism. Bar ADHD which has numerous amounts of literature validating NFTs efficacy, 40% of individuals with ASD have comorbid anxiety [24] that comprises both typical and autism-related anxiety [25].
Examples of autism-related anxiety are concerns about sensory stimuli, worries concerning change or unpredictable situations and uncommon but specific phobias. The following are examples of neurofeedback’s impact on anxiety:


-----
References
1. Coben R, Padolsky I. Assessment-guided neurofeedback for autistic spectrum disorder. Journal of Neurotherapy. 2007;11(1):5–23.
2. Baron-Cohen S. The Cognitive Neuroscience of Autism. Journal of Neurology, Neurosurgery & Psychiatry. 2004;75(7):945–8.
3. Coben R. Connectivity-Guided Neurofeedback for Autistic Spectrum Disorder. Biofeedback. 2007;35(4):131–5.
4. Neurofeedback Training to Improve Comprehension and Expression of ASD Child: A Case Study, Mutang and Bahari et al 2018
5. Faras H, Al Ateeqi N, Tidmarsh L. Autism spectrum disorders. Annals of Saudi Medicine. 2010;30(4):295–300.
6. Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, et al. Prevalence of autism spectrum disorder among children aged 8 years — autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2014. MMWR Surveillance Summaries. 2018;67(6):1–23.
7. Banerjee A, Miller MT, Li K, Sur M, Kaufmann WE. Towards a better diagnosis and treatment of Rett Syndrome: A model synaptic disorder. Brain. 2019;142(2):239–48.
8. Ellis MJ, Larsen K, Havighurst SS. Childhood disintegrative disorder (CDD): Symptomatology of the Norwegian patient population and parents’ experiences of patient regression. Journal of Autism and Developmental Disorders. 2021;52(4):1495–506.
9. Hamilton AF. Reflecting on the mirror neuron system in autism: A systematic review of current theories. Developmental Cognitive Neuroscience. 2013;3:91–105.
10. Ecker C, Bookheimer SY, Murphy DG. Neuroimaging in autism spectrum disorder: Brain structure and function across the lifespan. The Lancet Neurology. 2015;14(11):1121–34.
11. Mizuno A, Villalobos ME, Davies MM, Dahl BC, Müller R-A. Partially enhanced thalamocortical functional connectivity in autism. Brain Research. 2006;1104(1):160–74.
12. Turner KC, Frost L, Linsenbardt D, McIlroy JR, Müller R-A. Atypically diffuse functional connectivity between caudate nuclei and cerebral cortex in autism. Behavioral and Brain Functions. 2006;2(1).
13. Buxhoeveden D, Semendeferi K, Schenker N, Courchesne E. Decreased cell column spacing in autism. Social Neuroscience. 2004;582(6).
14. Just MA, Keller TA, Malave VL, Kana RK, Varma S. Autism as a neural systems disorder: A theory of frontal-posterior underconnectivity. Neuroscience & Biobehavioral Reviews. 2012;36(4):1292–313.
15. Konicar L, Radev S, Prillinger K, Klöbl M, Diehm R, Birbaumer N, et al. Volitional modification of brain activity in adolescents with autism spectrum disorder: A bayesian analysis of slow cortical potential neurofeedback. NeuroImage: Clinical. 2021;29:102557.
16. Kouijzer MEJ, de Moor JMH, Gerrits BJL, Congedo M, van Schie HT. Neurofeedback improves executive functioning in children with autism spectrum disorders. Research in Autism Spectrum Disorders. 2009;3(1):145–62.
17. Kouijzer MEJ, de Moor JMH, Gerrits BJL, Buitelaar JK, van Schie HT. Long-term effects of neurofeedback treatment in autism. Research in Autism Spectrum Disorders. 2009;3(2):496–501.
18. Mekkawy L. Efficacy of neurofeedback as a treatment modality for children in the autistic spectrum. Bulletin of the National Research Centre. 2021;45(1).
19. Datko M, Pineda JA, Müller RA. Positive effects of neurofeedback on autism symptoms correlate with brain activation during imitation and observation. European Journal of Neuroscience. 2017;47(6):579–91.
20. Friedrich EV, Sivanathan A, Lim T, Suttie N, Louchart S, Pillen S, et al. An effective neurofeedback intervention to improve social interactions in children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2015;45(12):4084–100.
21. Pineda JA, Carrasco K, Datko M, Pillen S, Schalles M. Neurofeedback training produces normalization in behavioural and electrophysiological measures of high-functioning autism. Philosophical Transactions of the Royal Society B: Biological Sciences. 2014;369(1644):20130183.
22. Holtmann M, Steiner S, Hohmann S, Poustka L, Banaschewski T, Bölte S. Neurofeedback in autism spectrum disorders. Developmental Medicine & Child Neurology. 2011;53(11):986–93.
23. van Hoogdalem LE, Feijs HM, Bramer WM, Ismail SY, van Dongen JD. The effectiveness of neurofeedback therapy as an alternative treatment for autism spectrum disorders in children. Journal of Psychophysiology. 2021;35(2):102–15.
24. Zaboski BA, Storch EA. Comorbid Autism Spectrum Disorder and anxiety disorders: A brief review. Future Neurology. 2018;13(1):31–7.
25. Lau BY, Leong R, Uljarevic M, Lerh JW, Rodgers J, Hollocks MJ, et al. Anxiety in young people with autism spectrum disorder: Common and autism-related anxiety experiences and their associations with individual characteristics. Autism. 2019;24(5):1111–26.
26. Liu S, Hao X, Liu X, He Y, Zhang L, An X, et al. Sensorimotor rhythm neurofeedback training relieves anxiety in healthy people. Cognitive Neurodynamics. 2021;16(3):531–44.
27. Salama AM, Abdel-Latif S, Omar T, El Wafa HA. Neurofeedback training and cognitive behavior therapy for treatment of generalized anxiety disorder in children and adolescents: A comparative study. NeuroRegulation. 2022;9(1):29–38.
28. Choi M-J, Park W-J. The effects of neurofeedback training on physical, psychoemotional stress response and self-regulation for late adolescence: A non-randomized trial. Journal of Korean Academy of Nursing. 2018;48(2):208.
29. Park W, Cho M, Park S. Effects of electroencephalogram biofeedback on emotion regulation and brain homeostasis of late adolescents in the COVID-19 pandemic. Journal of Korean Academy of Nursing. 2022;52(1):36.
30. Cowan J, Markam L. EEG biofeedback for the attention problems of autism: A case study. In 25th the Annual Meeting of the Association for applied Psychophysiology and Biofeedback. 1994Mar;
31. Coben R. Efficacy of connectivity guided neurofeedback for autistic spectrum disorder: controlled analysis of 75 cases with a 1 to 2 year follow-up. Journal of Neurotherapy. 2009; 13:81
32. Coben R, Middlebrooks M, Lightstone H, Corbell M. Four channel multivariate coherence training: Development and evidence in support of a new form of neurofeedback. Frontiers in Neuroscience. 2018;12.
33. DSM-5-TR: Autism spectrum disorder diagnosis [Internet]. Raising Children Network. 2022 [cited 2022Nov14]. Available from: https://raisingchildren.net.au/autism/learning-about-autism/assessment-diagnosis/dsm-5-autism-diagnosis
34. Meltzer JA, Negishi M, Mayes LC, Constable RT. Individual differences in Eeg Theta and Alpha Dynamics during working memory correlate with fmri responses across subjects. Clinical Neurophysiology. 2007;118(11):2419–36.
35. Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: Resting functional abnormalities in autism. Proceedings of the National Academy of Sciences. 2006;103(21):8275–80.





Comments